how to find number of valence electrons
So to get the number of electrons you must add the size of charge to the atomic or proton number. So to obtain that configuration the atoms either donated or rece.

Valence Electrons And Energy Levels Of Atoms Of Elements Video
Valence Electron Wikipedia

Which Lewis Electron Dot Diagram Is Correct For A S 2 Ion
To solve without a periodic table find the electron configuration of the element and count the electrons into 1 group of 2 and then into shells of 8.

How to find number of valence electrons in an ion.
Notethere is an order we fill the dots.
If we subtract 10 from 16 we get 6.
Valence electrons represented by the dots.
Now take this number and place a dot for each valence electron.
To learn how atoms form ions click here.
The electrons in the outer most energy level of an atom or ion.
The valence shell of ion may be empty mostly metals or fullnon metals.
Low state of energy is favored by every atom and lowest state of energy is duplet and octet configuration.
We can use this method to predict the charges of ions in ionic compounds.
Filling the electrons according to our rule we observe that the 21 st electron occupies the 3d sub shell.
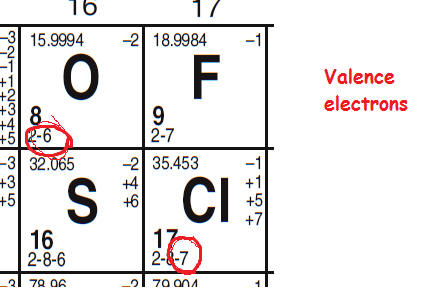
Oxygen gets 6 dots.
Chlorine gets 7 dots.
Furthermore the group number of the main group elements represents the number of valence electrons for that element in its ground state.
When forming ions elements typically gain or lose the minimum number of electrons necessary to achieve a full octet.
Oxygen is in the 16th period.
An elements proximity to the noble gases can help you keep track of its valence electrons in an ionic compound.
For example a group seven element has seven electrons in its valence shell.
It is the nucleus and the inner electrons of an atom or ion.
If the number is larger than 10 subtract 10 so you get two valence electrons.
For example fluorine has seven valence electrons so it is most likely to gain one electron to form an ion with a 1 charge.
If you look at the periodic table and at the period numbers that is the number of valence electrons.
Okay so now that we know how shells are filled we can move further to find the number of valence electrons in the transition elements.
The number in the last group is the amount of valence electrons.
The ones digit in the group number is the number of valence electrons.
Therefore oxygen has six valence electrons.
Consider scandium sc with its atomic number of 21.
So from figure 3 the number of electrons for chloride ion is 17 1 18 electrons.

Periodic Table

Counting Valence Electrons In A Molecule Or Polyatomic Ion Youtube
How Many Valence Electrons Does Oxygen Have

Ions Ppt Download

Valence Electrons And The Periodic Table Youtube
/85757530-56a12f0c5f9b58b7d0bcdabe.jpg)
Valence Definition In Chemistry
Valence Electrons And Lewis Electron Dot Of Atoms And Ions

Valence Electron Definition Configuration Example Video
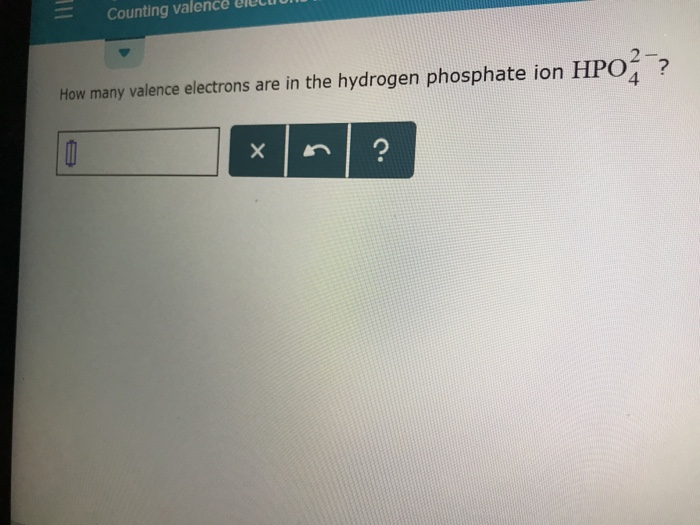
Solved Counting Valence Electr How Many Valence Electron

Valence Electron Wikipedia
What Is The Number Of Valence Electrons In A Sodium Ion Na

Valence Electrons Chemistry Socratic
What Are The Roles Of Valence Electrons In A Bond Quora

Drawing Dot Structures Video Khan Academy

Dublin Schools Lesson Ions How Do The Number Of Subatomic

How To Find Valence Electrons 12 Steps With Pictures Wikihow
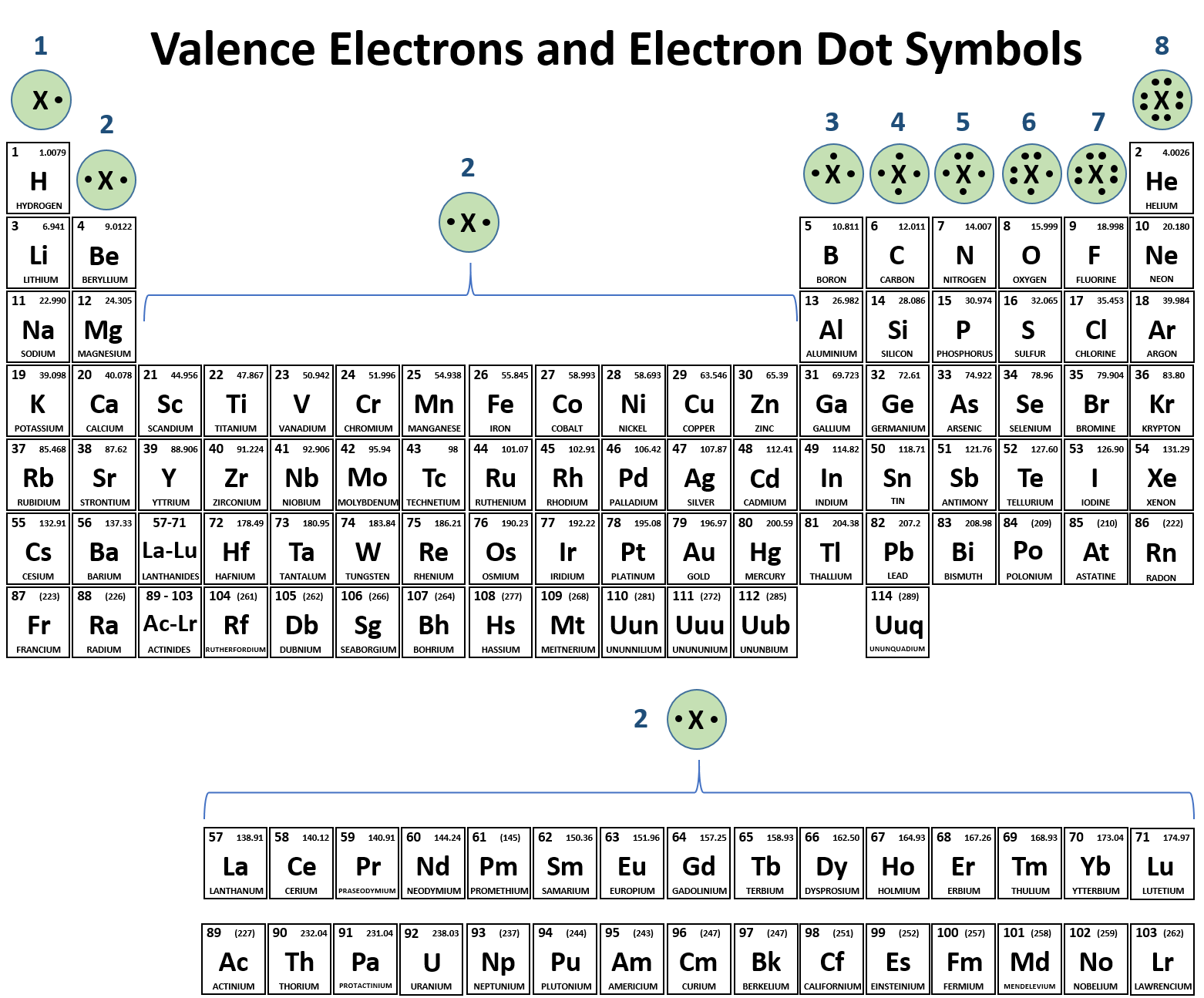
Ch104 Chapter 3 Ions And Ionic Compounds Chemistry
Solved How Many Valence Electrons In This Ammonium Ion I
how to find number of valence electrons
Source: https://goodttorials.blogspot.com/2019/07/how-to-find-number-of-valence-electrons.html
Posted by: doylecriall97.blogspot.com

0 Response to "how to find number of valence electrons"
Post a Comment